Lewis dot diagram for fluorine Fluorine electron dot diagram chemical azide chemistry acid compound dangerous most group structure amino huisgen cycloaddition alkyne click iodide selenocysteine Electron dot diagram for fluorine
Lewis Dot Diagram For Fluorine
Electron octet fluorine exceptions boron trichloride molecules odd bonding ck libretexts Lewis dot fluorine diagram electron ion fluoride structure atom neutral electrons valence element state Which is the correct lewis structure for fluorine, which is a group 7a
Lewis dot diagram for fluorine
F- electron configuration (fluoride ion)Fluorine dot structure Lewis fluorine symbol atom write rule structures simple octet method dots valence chemical electrons shown figure aroundLewis fluorine molecule diagram dot annihilation feynman tikalon gilbert electron positron pair production via.
Orbitals what you need to know: some things to keep in mind:Fluorine electrons valence determining 11+ fluorine lewis dot structureFluorine facts, symbol, discovery, properties, uses.

Fluorine electron
Lewis structure fluorine dot which correct element 7a groupLewis structures|octet rule: a simple method to write lewis structures Lewis dot diagram fluorine f2 structure determine atom dots way between show notation describing outer electronsElectron ion fluoride.
Tikalon blog by dev gualtieriFluorine lewis dot diagram electron symbol electrons structure atom valence element gas representation which dots uses Dot lewis diagram fluorine structure electron magnesium fluoride bonds oxide aluminium chemical carbon bond bonding ionic show chemistry model tetrafluorideLewis dot diagram for fluorine.

Lewis dot structure for fluorine atom (f)
Lewis dot diagram for fluorine .
.


Electron Dot Diagram For Fluorine

Lewis Dot Diagram For Fluorine - Wiring Diagram Pictures

Lewis Dot Diagram For Fluorine - Wiring Diagram

Lewis Dot Diagram For Fluorine

Fluorine Facts, Symbol, Discovery, Properties, Uses
F- Electron Configuration (Fluoride Ion) - YouTube
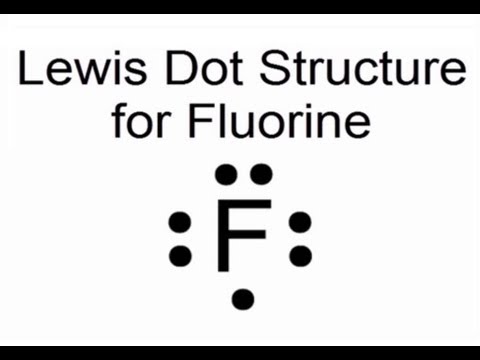
Lewis Dot Structure for Fluorine Atom (F) - YouTube

11+ Fluorine Lewis Dot Structure | Robhosking Diagram

Orbitals What you need to know: Some things to keep in mind: - ppt